research overview
WHEAT stands for WithHolding Enteral feeds Around packed red cell Transfusions. The WHEAT International trial is a randomized control trial exploring whether withholding enteral feeds (or milk feeds) around the time of blood transfusions will reduce the rate of necrotizing enterocolitis (NEC) in preterm babies. It is a multi-centre, comparative effectiveness trial which means that WHEAT International will compare two care practices already taking place to determine if one approach is more beneficial than the other to preterm babies. WHEAT International is currently taking place across the UK and Canada and will recruit an estimated 4500 babies over 4 years.
Necrotising enterocolitis (NEC) is a serious gut disease that affects about 1 in 20 very premature babies; about 1 in 3 will die of the disease and survivors are at high risk of long-term health and developmental problems.
Premature babies receive frequent feeds (every 1-3 hours) and they often need blood transfusions because they become anaemic (they do not have enough red blood cells). Some doctors worry that feeding babies during a blood transfusion may increase the risk of NEC. Others, however, think that it is more dangerous to stop feeds. Because of this, the way babies are cared for during a blood transfusion varies Internationally; some babies have feeds stopped before, during and after a transfusion (around 12 hours in total), and others have feeds continued.
The purpose of WHEAT International is to determine which approach is best. We will do this by comparing babies who have feeds stopped with those who have feeds continued during blood transfusions. Whether feeds will be stopped or continued will be decided by randomisation. Randomisation is done by computer and ensures that each baby has an equal chance of either having feeds stopped or continued.
FURTHER DETAILS
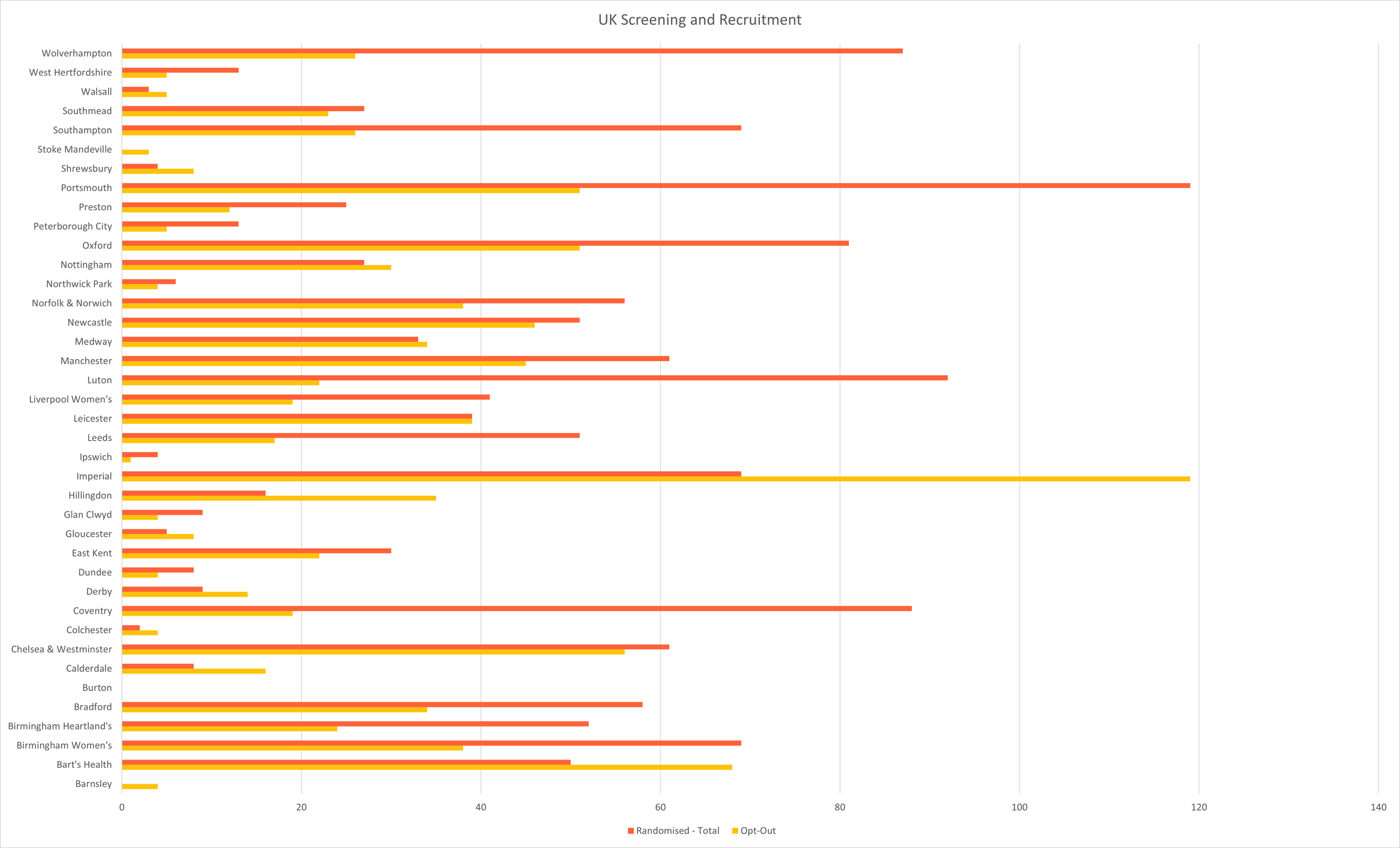
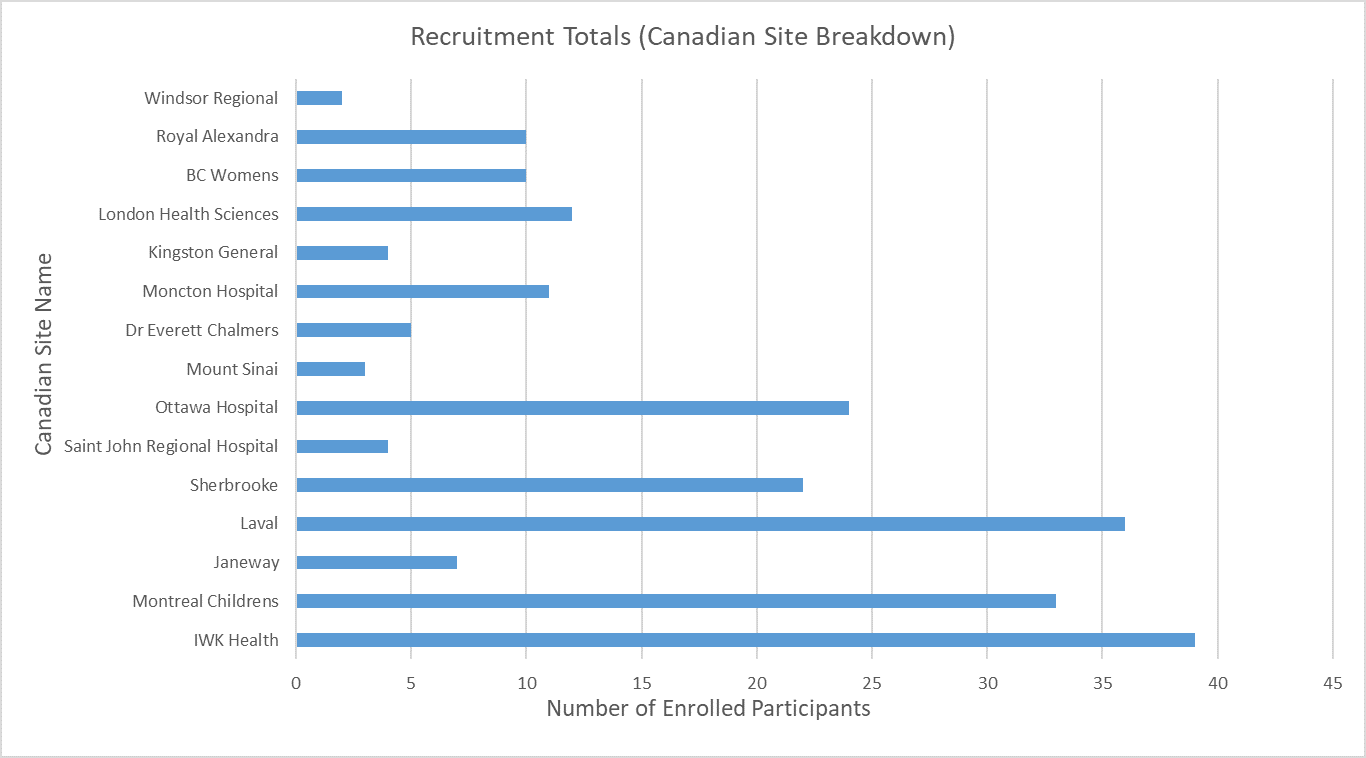
Screening & Recruitment
December 2025 | UK & Canada - Screening & Randomisation per site
Total Enrolled Infants: 1,651 (UK: 1,436; Canada: 215)
Last updated 08 Dec 2026
Target Enrollment (Global): 4333
Trial Information
Funder: Canadian Institutes of Health Research (CIHR)
Clinical Trials NCT: NCT05213806
Study Duration: Recruitment ending (planned) 31st December 2026